
Announcing a New Partnership with Abbott
CHCollective is excited to announce a new partnership with Abbott. As a global leader in rapid testing, Abbott’s point-of-care testing technology provides actionable results in minutes, helping health center clinicians determine the most appropriate care pathway while patients are being seen. Abbott and their product portfolio span key diagnostic testing areas such as infectious disease, cardiovascular disease, diabetes, and connectivity solutions.
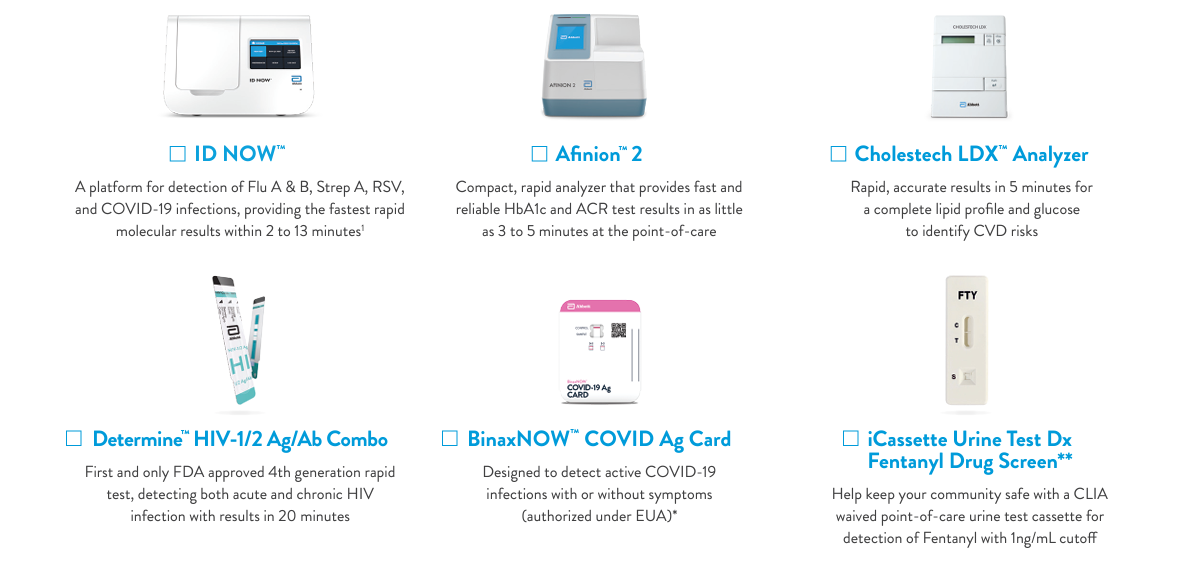
Abbott Also Announces a New Health Center-Specific Program!
Coinciding with the CHCollective partnership, Abbott also now offers cost-effective, accurate solutions for Infectious Disease, Cardiometabolic, HbA1c, and Toxicology specific to health centers! Abbott’s reliable, fast tests lead to optimal care and patient satisfaction.
Learn more about this program and how Abbott helps health centers do what they do best: care for the community.
* The BinaxNOW™ COVID-19 Ag Card has not been FDA cleared or approved. It has been authorized by the FDA under an emergency use authorization. It has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens, and is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner
